Progress in quest for MTM treatment (Sep. 24)
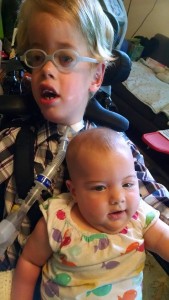
Last Thursday the biotech company Audentes — which has taken the lead on developing a treatment for myotubular myopathy (MTM) — announced that the first ever human patient had been given a dose of AT132, a form of gene therapy. This is a huge development for the community of families we’ve gotten to know through MTM, the condition that Lucas was born with. This first clinical trial for human patients (called “ASPIRO”) comes after clinical trials using dogs diagnosed with MTM; in that trial the affected dogs had significantly increased muscle strength after a series of doses using the same gene therapy model.
The press release goes on to say, “ASPIRO is a multicenter, multinational, open-label, ascending dose study to evaluate the safety and preliminary efficacy of AT132 in approximately twelve XLMTM patients less than five years of age. Preliminary data from ASPIRO is expected to be available in the fourth quarter of 2017.”
 There is a lot of excitement about this development, and certainly it’s been a long time in coming. Many parents, researchers, doctors, foundations, and other supporters have done tremendous work to bring us to the point of having this complex treatment tested on children. We’ve been fortunate to get to know some of these people through the MTM family conference that we attended and through contact with one of the doctors who happens to be based at the University of Washington.
There is a lot of excitement about this development, and certainly it’s been a long time in coming. Many parents, researchers, doctors, foundations, and other supporters have done tremendous work to bring us to the point of having this complex treatment tested on children. We’ve been fortunate to get to know some of these people through the MTM family conference that we attended and through contact with one of the doctors who happens to be based at the University of Washington.
I don’t pretend to totally get the science of gene therapy, but it’s been something that disease researchers have imagined could bring profound changes to many people effected by rare and severe conditions like MTM. The hope for gene therapy has in many ways not materialized, so success with MTM would be a significant breakthrough. As I understand it, the model being developed by Audentes basically uses a virus to introduce the non-mutated MTM1 gene into the cells of kids who live with MTM. The MTM1 mutation is what inhibits the development of a protein called myotublarin, and without myutubularin, muscle cells don’t develop properly. So by essentially “correcting” the MTM1 mutation, myutubularin would suddenly be developed in the body, leading to increased muscle strength and tone.
Dr. Casey Childers, who we’ve gotten to know because he’s based here in Seattle, talks about the success of the model with dogs in this short video. At the end you can actually see the remarkable changes to the dogs based on just a few months of treatment:
Krista and I have written in the past about some of our ambivalence related to the drive for a MTM “cure.” Again, this doesn’t mean that we’re not grateful for all that has been done, nor are we dismissive of the ways that gene therapy could potential make Lucas’s life easier. The prospect of him not having to use a ventilator to breathe someday, for example, would be truly transformational for his life and for our lives.
For me, the ambivalence has to do with the intense love I feel for my son– just as he is now. MTM is part of Lucas, it always has been and always will be. There is no way to undo the effects that severe muscle disease have had on Lucas; he will always use a wheelchair, always have slurred speech, and likely always have to consume most of his food through a tube. But more importantly, his joyous personality, quirky sense of humor, indomitable spirit – these things that I treasure about Lucas are also directly related to his experience of growing up with MTM. A treatment to improve his health and quality of life – yes, if it comes to pass during his lifetime we would welcome it. But in my mind the idea of a “cure” remains problematic.
I guess that’s why upon seeing the news my thought was “cool, I should write a blog post about that.” There is, of course, also the question of whether it will actually work. Before we get our hopes up too high we have to acknowledge that something that worked in dogs may not work the same way in humans. And there other unknowns, like detrimental side effects, high costs that aren’t covered by insurance, the number of years it will take to move from an initial clinical trail on twelve kids to having a FDA approved treatment on the market, etc. And then there are the larger ethical questions around gene therapy: who gets to decide what is “normal” and what disabilities should be “fixed”; if gene therapy becomes common someday, will society be even less accepting of people with disabilities; will it only be available to those able to afford it; and so on.
So we’ll wait and see what happens when results of these early tests are released in January. We’ll continue loving and reveling in Lucas just as he is, while cautiously wondering about and hoping for a time when his muscle disease could be made less severe.